Europe and Asia, becoming less frequent northwards. A smaller centre occurs on the Pacific side of North America. The order is less developed in the south temperate zone.
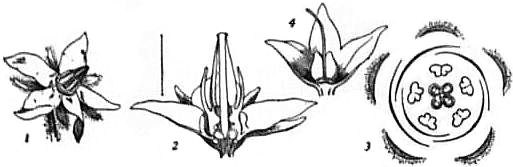
Fig. 3.—(1) Flower of Borage; (2) same in vertical section enlarged; (3) horizontal plan of flower; (4) flower of Comfrey after removal of corolla, showing unripe fruit.
The order is of little economic value. Several genera, such as borage and Pulmonaria, were formerly used in medicine, and the roots yield purple or brown dyes, as in Alkanna tinctoria (alkanet). Heliotrope or cherry-pie (Heliotropium peruvianum) is a well-known garden plant.
BORÅS, a town of Sweden, in the district (län) of Elfsborg,
45 m. E. of Gothenburg by rail, on the river Viske. Pop. (1880)
4723; (1900) 15,837. It ranks among the first twelve towns
in Sweden both in population and in the value of its manufacturing
industries. These are principally textile, as there are
numerous cotton spinning and weaving mills, together with a
technical weaving school. The town was founded in 1632 by
King Gustavus Adolphus.
BORAX (sodium pyroborate or sodium biborate), Na2B4O7,
a substance which appears in commerce under two forms,
namely “common” or prismatic borax, Na2B4O7·10H2O, and
“jewellers’ ” or octahedral borax, Na2B4O7·5H2O. It is to be
noted that the term “borax” was used by the alchemists in a
very vague manner, and is therefore not to be taken as meaning
the substance now specifically known by the name. Prismatic
borax is found widely distributed as a natural product (see below,
Mineralogy) in Tibet, and in Canada, Peru and Transylvania,
while the bed of Borax Lake, near Clear Lake in California,
is occupied by a large mass of crystallized borax, which is fit
for use by the assayer without undergoing any preliminary
purification. The supply of borax is, however, mainly derived
from the boric acid of Tuscany, which is fused in a reverberatory
furnace with half its weight of sodium carbonate, and the mass
after cooling is extracted with warm water. An alternative
method is to dissolve sodium carbonate in lead-lined steam-heated
pans, and add the boric acid gradually; the solution
then being concentrated until the borax crystallizes. Borax
is also prepared from the naturally occurring calcium borate,
which is mixed in a finely divided condition with the requisite
quantity of soda ash; the mixture is fused, extracted with water
and concentrated until the solution commences to crystallize.
From a supersaturated aqueous solution of borax, the pentahydrate, Na2B4O7·5H2O, is deposited when evaporation takes place at somewhat high temperatures. The same hydrate can be prepared by dissolving borax in water until the solution has a specific gravity of 1.246 and then allowing the solution to cool. The pentahydrate is deposited between 79° C. and 56° C.; below this temperature the decahydrate or ordinary borax, Na2B4O7·10H2O, is deposited. Crystals of ordinary borax swell up to a very great extent on heating, losing their water of crystallization and melting to a clear white glass. The crystals of octahedral borax fuse more easily than those of the prismatic form and are less liable to split when heated, so that they are preferable for soldering or fluxing. Fused borax dissolves many metallic oxides, forming complex borates which in many cases show characteristic colours. Its use in soldering depends on the fact that solder only adheres to the surface of an untarnished metal, and consequently a little borax is placed on the surface of the metal and heated by the soldering iron in order to remove any superficial film of oxide. It is also used for glazing pottery, in glass-making and the glazing of linen.
Boric acid (q.v.) being only a weak acid, its salts readily undergo hydrolytic dissociation in aqueous solution, and this property can be readily shown with a concentrated aqueous solution of borax, for by adding litmus and then just sufficient acetic acid to turn the litmus red, the addition of a large volume of water to the solution changes the colour back to blue again. The boric acid being scarcely ionized gives only a very small quantity of hydrogen ions, whilst the base (sodium hydroxide) produced by the hydrolysis occasioned by the dilution of the solution, being a “strong base,” is highly ionized and gives a comparatively large amount of hydroxyl ions. In the solution, therefore, there is now an excess of hydroxyl ions; consequently it has an alkaline reaction and the litmus turns blue.
Mineralogy.—The Tibetan mineral deposits have been known since very early times, and formerly the crude material was exported to Europe, under the name of tincal, for the preparation of pure borax and other boron salts. The most westerly of the Tibetan deposits are in the lake-plain of Pugha on the Rulangchu, a tributary of the Indus, at an elevation of 15,000 ft.: here the impure borax (sohaga) occurs over an area of about 2 sq. m., and is covered by a saline efflorescence; successive crops are obtained by the action of rain and snow and subsequent evaporation. Deposits of purer material (chú tsalé or water borax) occur at the lakes of Rudok, situated to the east of the Pugha district; also still farther to the east at the great lakes Tengri Nor, north of Lhasa, and several other places. More recently, the extensive deposits of borates (chiefly, however, of calcium; see Colemanite) in the Mohave desert on the borders of California and Nevada, and in the Atacama desert in South America, have been the chief commercial sources of boron compounds. The boron contained in solution in the salt lakes has very probably been supplied by hot springs and solfataras of volcanic origin, such as those which at the present day charge the waters of the lagoons in Tuscany with boric acid. The deposits formed by evaporation from these lakes and marshes or salines, are mixtures of borates, various alkaline salts (sodium carbonate, sulphate, chloride), gypsum, &c. In the mud of the lakes and in the surrounding marshy soil fine isolated crystals of borax are frequently found. For example, crystals up to 7 in. in length and weighing a pound each have been found in large numbers at Borax Lake in Lake county, and at Borax Lake in San Bernardino county, both in California.
Borax crystallizes with ten molecules of water, the composition of the crystals being Na2B4O7 + 10H2O. The crystals belong to the monoclinic system, and it is a curious fact that in habit and angles they closely resemble pyroxene (a silicate of calcium, magnesium and iron). There is a perfect cleavage parallel to the orthopinacoid and less perfect cleavages parallel to the faces of the prism. The mineral is transparent to opaque and white, sometimes greyish, bluish or greenish in colour. Hardness 2–212; sp. gr. 1.69–1.72.
The optical characters are interesting, because of the striking crossed dispersion of the optic axes, of which phenomenon borax affords the best example. The optic figure seen in convergent polarized light through a section cut parallel to the plane of symmetry of a borax crystal is symmetrical only with respect to the central point. The plane of the optic axes for red light is inclined at 2° to that for blue light, and the angle between the optic axes themselves is 3° greater for red than for blue light.
BORDA, JEAN CHARLES (1733–1799), French mathematician
and nautical astronomer, was born at Dax on the 4th of May 1733.
He studied at La Flèche, and at an early age obtained a commission
in the cavalry. In 1756 he presented a Mémoire sur le mouvement des projectiles to the Academy of Sciences, who elected
him a member. He was present at the battle of Hastembeck,
and soon afterwards joined the naval service. He visited the
Azores and the Canary Islands, of which he constructed an
admirable map. In 1782 his frigate was taken by a British
squadron; he himself was carried to England, but was almost
immediately released on parole and returned to France. He
died at Paris on the 20th of February 1799. Borda contributed
a long series of valuable memoirs to the Academy of Sciences.
His researches in hydrodynamics were highly useful for marine
engineering, while the reflecting and repeating circles, as improved
by him, were of great service in nautical astronomy.
He was associated with J. B. J. Delambre and P. F. A. Méchain
in the attempt to determine an arc of the meridian, and the
greater number of the instruments employed in the task were
invented by him.
See J. B. Biot, “Notice sur Borda” in the Mém. de l’Acad. des Sciences, iv.
BORDAGE. (1) A nautical term (from Fr. bord, side) for the planking on a ship’s side. (2) A feudal term (from Lat. borda, a cottage) for the tenure by which a certain class of villein held